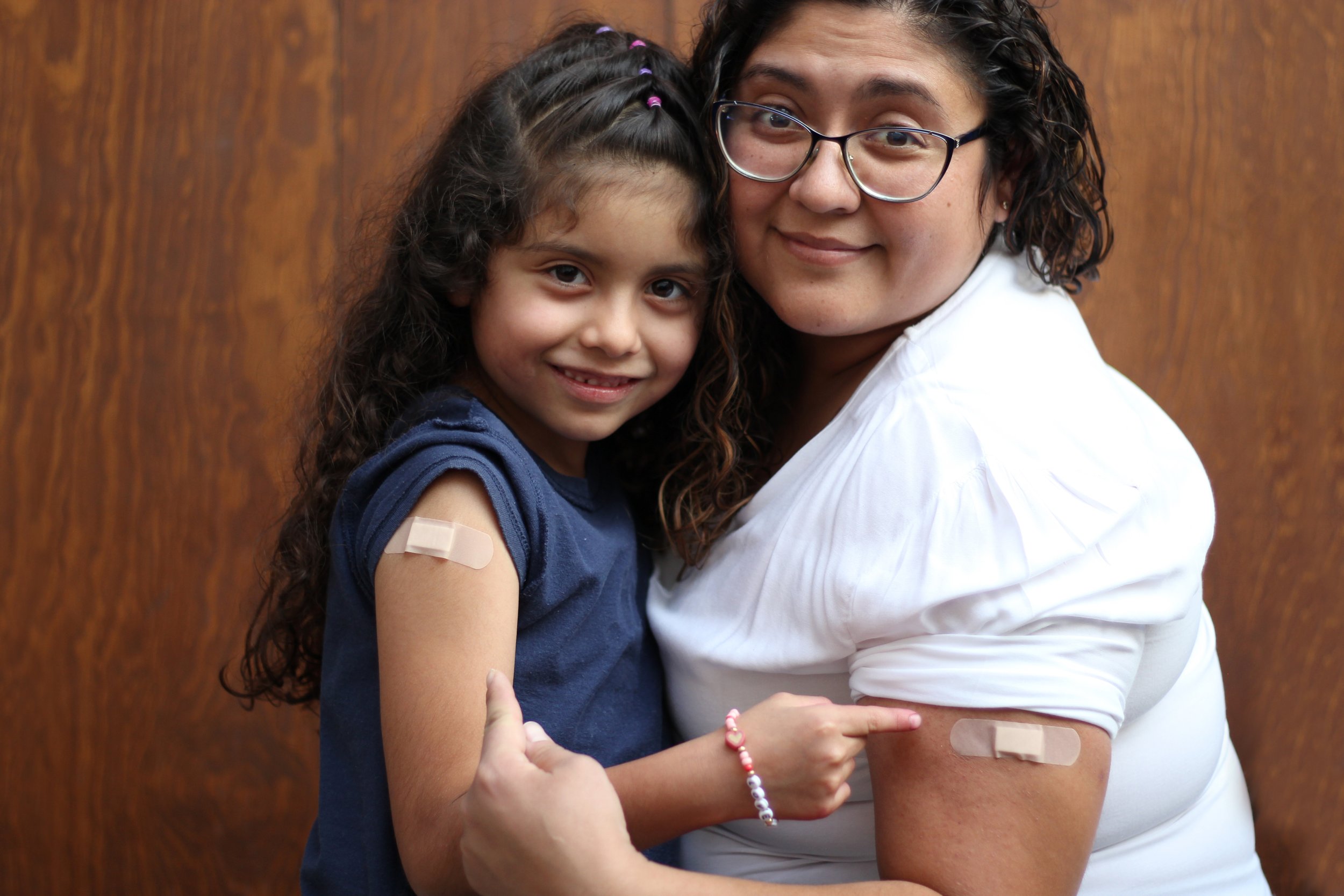Over the past two years, our list of questions about COVID-19 has grown immensely. As parents, we are looking for the most up to date information from trustworthy sources to help make the best decisions for our families’ health.
Earlier this summer, infant and toddler COVID-19 vaccines were granted emergency use authorization by the FDA after comprehensive clinical trials showed the COVID-19 vaccines to be safe and effective for those over the age of 6 months. This new recommendation makes roughly 2.2 million children in California and nearly 20 million children in the US younger than 5 years of age eligible for the COVID-19 vaccine.
Last week, Bricia and Paulina sat down with Dr. Erica Pan, State Epidemiologist and mom of two, to ask her questions that many of us are thinking.
Our Interview with Dr. Erica Pan
Q: Are children receiving the same dosage as adults?
A: The different drug manufacturers have their own formulas for their COVID-19 vaccine, and their dosages reflect what a person needs to gain protection from the virus with these safe and effective vaccines.
The dosages are based on a child’s average size at a certain age. Doctors have found the right dosage for children that show the same amount of antibody responses that adult dosages have provided.
Q: What does the vaccine dose schedule look like for kids?
A: Moderna is a two-dose COVID-19 vaccination for kids ages 6 months to 5 years and for ages 6-17. The two doses are given at least 28 days apart.
Pfizer is a three-dose vaccine for kids ages 6 months to 4 years (5-17 already authorized). The first two doses are given at least 21 days apart, with the third dose given at least 60 days later.
Q: Is there a better vaccine choice for our children? Pfizer or Moderna?
A: It is important to remember that COVID-19 vaccines remain the safest way to prevent hospitalization and death from the disease for everyone 6 months and older – no brand is better than the other.
Q: What do the clinical trials for the children’s vaccine entail?
A: Clinical trials involving thousands of infants and toddlers 6 months and older firmly show that the COVID-19 vaccines are safe and effective in this population.
Pfizer vaccine trials involved roughly 4,500 infants and toddlers while Moderna vaccine trials involved over 6,500 infants and toddlers all over the age of 6 months. The scientific review found that completion of either vaccine series produced antibody levels similar to those achieved in individuals 16-25 years.
With their parent’s permission, children are enrolled in the trial randomly. They either receive a placebo (saltwater) or the vaccine. The children receive regular blood tests and are tested for how much their immune systems respond to the vaccine. Doctors check the levels of the antibodies over time to learn about overall effectiveness of the vaccine. Any side effects to a vaccine would typically present themselves within the first month or two after receiving the vaccine. This is typical of any vaccine.
Q: Where can parents take their children to receive their COVID-19 vaccines?
A: Families can schedule an appointment for their children to receive a COVID-19 vaccine in select pharmacy locations. By law, pharmacies can independently vaccinate children 3 and older and can vaccinate children under 3 if they work in partnership with another provider. However, this varies by pharmacy. Contact your local pharmacy to see which ages they vaccinate.
Call your pediatrician or health clinic to get your child vaccinated or, for readers in California, go to MyTurn.ca.gov or call the hotline at 833-422-4255 to find a vaccine near you.
You can listen to the rest of our conversation with Dr. Pan on one of our most recent podcast episodes. This episode and blog post are brought to you by the CA Department of Public Health
More about Dr. Erica Pan: Erica Pan, MD, MPH, FAAP, the Deputy Director of the Center for Infectious Diseases and State Epidemiologist, was sworn in July 13, 2020. Prior to joining CDPH, Pan served in several positions at both the Alameda County Public Health Department since 2011, and the San Francisco Department of Public Health from 2004 – 2011. She is also a Clinical Professor in the Department of Pediatrics, Division of Pediatric Infectious Diseases at the University of California, San Francisco. She maintained her clinical work at San Francisco General Hospital and at UCSF Benioff Children's Hospitals in San Francisco and Oakland in Infectious Diseases until 2019.